Your patients will
Feel
the Ferralet® 90 difference
Formulated with your patients’ comfort in mind.
Ferralet® 90 Savings
You may be eligible to save money on your prescription by
using the Ferralet® 90 Savings Card.*
To redeem your savings card:
- Present your prescription, along with the savings card, at your participating pharmacy.
- Follow your doctor’s instructions on how to take Ferralet 90 and enjoy the nutritional goodness!
*Subject to eligibility. Terms and conditions apply.
To redeem your savings card:
- Present your prescription, along with the savings card, at your participating pharmacy.
- Follow your doctor’s instructions on how to take Ferralet 90 and enjoy the nutritional goodness!
*Subject to eligibility. Terms and conditions apply.
Is Ferralet® 90 indicated for all anemias?
Ferralet® 90 is indicated for the treatment of all anemias that are responsive to oral iron therapy (for instance: hypochromic anemia associated with pregnancy, chronic and/or acute blood loss, metabolic disease, postsurgical convalescence, and dietary needs).1
Ferralet® 90 should be taken as a once-daily tablet, two hours after a meal. As such, some patients prefer bedtime dosing. Tablets should not be chewed.1
Two complimentary iron sources
Does Ferralet® 90 offer fewer gastrointestinal (GI) side effects?
GI side effects are a common problem associated with oral iron therapy.2
Ferralet® 90 with Ferr-Ease® was formulated to make iron therapy as gentle and comfortable as possible for your patients.2


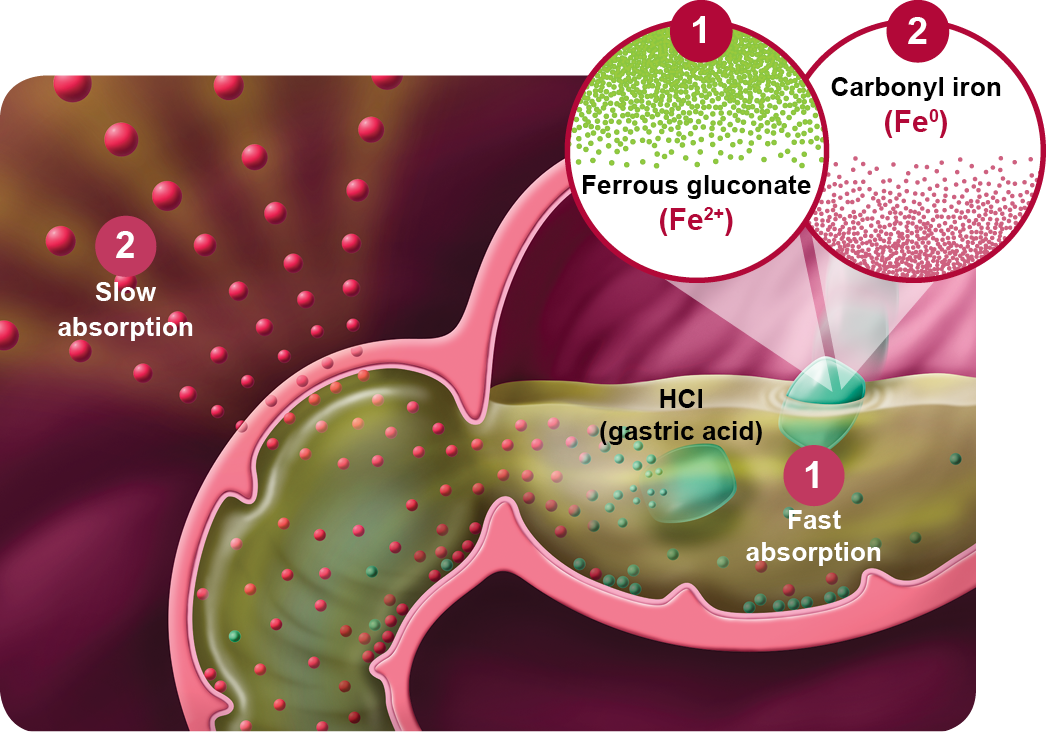
Dual-iron for complementary absorption
- Ferrous gluconate (Fe2+) enters the body in the ferrous state, ready for quick and optimal dissolution and absorption to start restoring your patients’ vital iron levels.3
- Particulate carbonyl iron (Fe0) requires conversion from its elemental form to the soluble ionized form (Fe0+ 2H+Cl–→Fe2+Cl2 + H2). It is naturally regulated by the patients’ production of gastric acid for gradual absorption.4 Overall, the bioavailability of carbonyl iron is similar to ferrous salts but carbonyl iron has less GI side effects.4

30-70% of patients on oral iron supplements report GI side effects, which may lead to poor adherence to treatment.2
DID YOU KNOW?
Did you know that Ferralet® 90 contains a gentle and effective stool softener (50 mg docusate sodium) that helps prevent constipation that might occur in patients sensitive to iron therapy.5


What is the Ferralet® 90 wholesale information?
- NDC 0178-0089-90
- Packaged in bottles of 90
- Film-coated tablets
| Wholesaler | Order Entry Numbers |
|---|---|
| AmerisourceBergen | 10008969 |
| Cardinal Health, Inc | 4278834 |
| McKesson Corp | 1600634 |
| Morris & Dickson Co. | 028886 |
| NC Mutual Wholesale Drug | 777276 |
| Smith Drug | 634196 |
| Valley Wholesales | 378927 |
| Value Drug Co. | 497040 |
- Ferralet 90 Prescribing Information. San Antonio, TX: Mission Pharmacal Company.
- Muñoz M, Gómez-Ramírez S, Bhandari S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf. 2018;17(2):149-159.
- Beck KL, Conlon CA, Kruger R, et al. Dietary determinants of and possible solutions to iron deficiency for young women living in industrialized countries: a review. Nutrients. 2014;6(9):3747-3776.
- Huebers HA, Brittenham GM, Csiba E, et al. Absorption of carbonyl iron. J Lab Clin Med. 1986;108(5):473-478.
- Medscape. Docusate (OTC). http://reference.medscape.com/drug/colace-dss-docusate-342012. Accessed May 2022..




